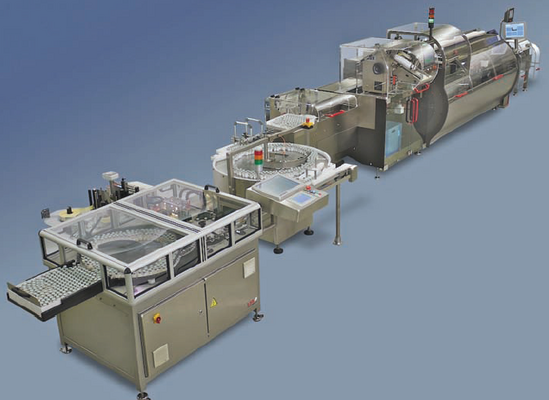
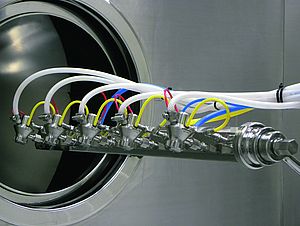
In order to meet its customers’ diversified requirements, Axellia recently began producing finished dosage forms of APIs into injectables. This enables the company to offer both raw APIs as well as finished products in an injectable format. Axellia’s existing filling line for injectables was exclusively devoted to powder filling, which was limited to only a few API products. To expand capacity for finished injectables and extend their portfolio to different API products, Axellia sought a liquid filling solution with sterile processing and lyophilisation capabilities. Due to the unstable nature of certain API products, they must be freeze-dried into dry powder form after being filled into injectable vials to ensure a reasonable shelf life of the finished product. Axellia ordered a complete filling line in the summer of 2007, consisting of a washing machine, sterilisation tunnel, filling and sealing machine, and an overseal capping machine. To meet the unique requirements of this project, the FLC 3080 filling and sealing machine was chosen, which offers time-pressure filling, automatic cleaning-in-place (CIP) and sterilising-in-place (SIP) systems, and an automatic checkweighing system. Axellia specified the time-pressure filling system, instead of the traditional pump filling system, because it is easier to validate the CIP and SIP process. There are less mechanical parts in the time-pressure filling system compared to pump filling system, thus minimising maintenance and offering an easier cleaning and sterilisation process. The automatic CIP/SIP system feature of the machine offers some critical benefits to Axellia. It covers the in-line integrated sterilizing filter so there is no need to dismantle the system. The system complies with GMP regulations, minimis-ing production times and eliminating the danger of product contamination. The fully automated, non-destructive checkweighing in-process control system ensures the best product quality control. With a speed of 300 vials per minute, the filling machine runs on an eight-hour shift. The checkweighing system controls eight random samples every three minutes and incorrect dosage amounts are automatically rejected without process interruptions. At the same time, the checkweighing system gives real-time in-process feedback to the time-pressure filling to ensure accurate dosing. This fully automated system reduces the necessity of having quality insurance engineers on site, thus reducing labour costs and minimising total costs of ownership. Uniquely for Axellia, the checkweighing inprocess control offers a tracking system designed in cooperation with the supplier of the freeze driers. This tracking system provides direct communication between the filling machine, the freeze driers and the capping machine, which ensures that incorrect vials will be automatically rejected even after the freeze-drying process. The whole automatic process and communication gives Axellia further quality assurance. When the complete filling line was delivered in the summer of 2008, the Project manager at Axellia, Mr. Knud-E Andersen, was impressed with the on-time delivery and the overall quality of the system. At the same time, he was also looking for an end-of-line secondary packaging solution to package the vials into cartons. Again Axellia turned to Bosch for a solution. For the end-of-line packaging, Axellia needed a labelling machine, a turntable, a cartoning machine, checkweigher, stretch bander and a case packer. However, Axellia didn’t want to source these individual modules from different suppliers. Bosch was able to offer a customised one-stop-shopping solution by sourcing the labelling machine, stretch bander and case packer from outside vendors and integrating them with its own cartoning machine and checkweighing unit. Thus, Bosch provided Axellia with a complete end-of-line packaging solution together with on-site installation and support. This service and support gives Mr. Knud-E Andersen the convenience of interacting with only one supplier and makes the whole line integration and validation process easier. The CUC cartoning machine offers excellent accessibility. The servo technology, industrial PC control systems and the ergonomic touch screen operating panel provide for secure, reliable and trouble-free production runs. The product insertion from the back of the machine significantly reduces maintenance time and provides for easier cleaning and trouble-shooting. The encapsulation of the drive components and the slanted guards considerably reduce the time required for line clearance. The modular checkweighing system KWE 4000 meets pharmaceutical safety and cGMP requirements, as well as offering simple operation and easy line integration. The control cabinet at the rear of the checkweigher and the operator terminal allows excellent accessibility. Production statistics, trend curves and current weight are displayed on the standard screen which can be configured to show further information if required. The weight display can be altered to large numbers for remote readability by one single touch on the screen. “I have had a good experience with Bosch. We believe in Bosch, that’s why we buy it from Bosch,” said Mr. Knud-E Andersen.
Compact System Offers Filling and Packaging
Axellia is a leading producer of fermented anti-infective active pharmaceutical ingredients (API) and injectable finished products.
- by Syntegon Technology GmbH
- May 6, 2009
- 260 views