Traditionally, compressed air has had several shortcomings, impacting drug manufacturing and handling, such as high maintenance cost, high risk of contamination and the presence of moisture. Compressed air quality in a pharmaceutical manufacturing process has a direct relation to the end-product quality. The luxury afforded previously by pharmaceutical companies was that this quality was worth paying the additional price. Nowadays, pharmaceutical companies need to balance the rising costs and energy emissions of the plant while at the same time maintaining product quality and process efficiency. This means that they start to challenge the existing norms and look for innovative solutions to their quality compressed air needs.
In addition, reducing emissions and energy use of operations is also under the increased attention of policymakers. In Europe, energy efficiency and the broader goal of reduction in carbon emissions add to the pressures of the manufacturing industries. Regardless of the current pandemic and anticipated global slowdown, the European Commission’s flagship initiative (the European Green Deal), remains a key priority, which aims to “transform the EU into a fair and prosperous society, with a modern, resource-efficient and competitive economy”. Successful and economically sustainable businesses know that they need to make the investment decisions now to protect their viability in the medium-term. Responsibility lies with industry to play its part in making the EU carbon neutral by 2050, which includes meeting the interim target of a 55% reduction in CO2 emissions by 2030.
Air purity
For a pharmaceutical manufacturing plant, compressed air is one of the utilities of absolute priority. The absence of compressed air will bring a plant to a standstill, much like the lack of power. However, the lack of quality air, which does not meet the global compliance requirements of ISO 8573, 1:2010 and ISO 8573-7, can cause a high risk of contamination and moisture. This could result in drug recalls and export bans, thereby damaging the reputation of the company in question. Across these processes, plants must maintain zero tolerance for impurities.
Oil free screw compressors are the preferred choice as they compress the air inside an oil free compression chamber, which is well sealed to avoid lubrication oil contaminating the compressed air.
Until a few years ago, companies used oil injected compressors with filtration arriving to ‘Class 1’ oil quality standards, which means the air must have no more than 0.01 mg/m3 of oil residue. To ensure no residual oil, airborne particulates or vapour could enter the system, they used downstream air dryers and double line filters to further purify the air.
Today, quality sensitive industries such as drug manufacturing use oil free air compressors that deliver ISO:8573 (P-2):2007 ‘Class 0’ oil free air to ensure 100% contaminant-free air is produced for various applications in their manufacturing and packaging processes. However, until recently, these compressors were expensive and were used primarily by large companies and those that exported drugs to markets with stringent standards (ex., the US Food and Drug Administration). With the advanced oil free technology in ‘Class 0’ certified compressors, every pharmaceutical manufacturing company is assured of not only oil free air that meets the most stringent standards, but also much higher energy savings and uptime.
No oil. Water
Compliance and quality norms clearly define the pharma industry thereby pushing compressor manufacturers to evolve and demonstrate their commitment to compressed air purity of the highest standards. Very often the pursuit for purity of compressed air brings pharma manufacturers to extremes. For example, installing multiple filters instead of one, which increases energy use and drives complexity resulting in increased maintenance and costs.
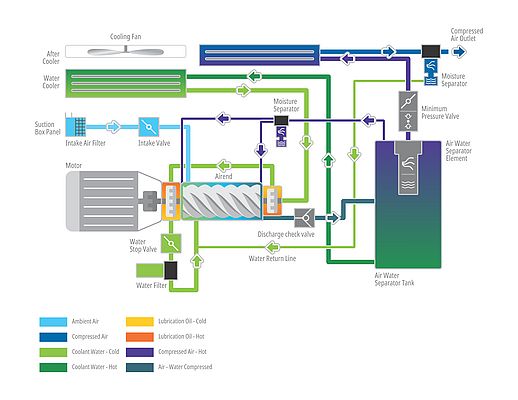
For several years, the pharmaceutical industry has seen water injected oil free compressors as a highly efficient alternative to traditional oil-free, dry screw two-stage compressors. It delivers the same ‘Class 0’ compressed air in a much simpler, one stage design whereby water is injected into the air-end as opposed to oil thereby cooling it down and sealing the system. Consequently, the compressor and air-end are running at a much lower speed than a dry screw compressor, which translates in increased reliability, lower parts wear, less maintenance and better energy efficiency. There is just one “but”. Most of the water injected oil-free compressors come with additions like sensitive reverse osmosis (RO) purifiers in the water inlet line, special rotor materials and complex bearing systems. These systems have proven to be cost intensive and prone to failure.
Oil free and water injected. Disrupted
Today, there is an answer to these issues:
- a closed loop water circuit design with less complexity
- no special bearings or exotic materials
- no need for sensitive RO purifying systems
In short, an innovative and much more reliable design. For pharmaceutical manufacturing where failures equal significant losses, and where reduced power and maintenance costs are critical, the advantages are clear.
In this closed-circuit system, the risk of contamination is lowered even further. The water is topping up; rinsing and cleaning the insides of the compressor. Furthermore, the compressor drains the condensate wastewater which doesn’t require any additional treatment (as it’s of drinking water quality).
Finally, in addition to cleaner water, and the slower running of the compressor, the closed-loop system can rely on standard, more cost-efficient bearings as used in traditional oil lubricated compressors, reducing complexity and costs further.
The total cost of ownership of this water-injected compressor is considerably lower (8% or more) versus traditional two stage oil free screw compressors thanks to the reduced power consumption and the simplicity of the design as well as maintenance ease. Compared with oil injected solutions, these systems are even more interesting since the downstream filtration is less stressed.
Simplicity, efficiency, and having lower maintenance requirements, the advanced design language of these latest closed loop water-injected oil-free compressors can help companies unlock new avenues of quality in their compressed air needs while also improving equipment peak uptime. All this translates into the energy consumption and reliability costs of a single stage oil lubricated compressor, while providing an oil free solution. This in turn protects the pharmaceutical manufacturing processes against more stringent energy efficiency regulations while meeting Class 0 air quality standards - all for a lower total lifecycle cost.