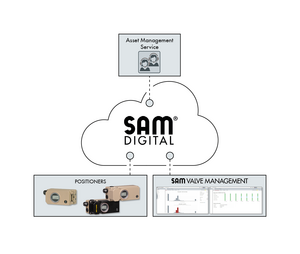
Control valves with smooth, cavity-free internals which help to promote effective CIP and SIP (Clean or Sterilised in Place) and which are also self-draining, are the preferred choice in many food processing and pharmaceutical applications. Samson offers a range of valves incorporating these features which are manufactured to quality assurance systems certified to ISO 9001 and which also meeting stringent FDA requirements for aseptic process applications.
For example, the type 3347 control valve has polished steel parts to ensure the highest purity for the process medium by eliminating areas which might increase the potential for bacteria growth such as traps and crevices. They also feature special PTFE bushing and an additional steam line connection that is used to meet the highest purity requirements and prevents bacteria from spreading at the actuator stem guide.
Another example is the Type 3249 valve which is equipped with an EPDM diaphragm and a back-up safety packing box to ensure total protection against bacteria spreading and prevent product leakage. These sealing systems, which constitute the main components of the valve construction, have been thoroughly developed and designed to ensure a long and reliable service life.
Finally, process applications which involve pasty, fibrous or highly viscous products can be controlled by a Type 3345 cavity-free valve body which features a diaphragm made of either; rubber, nitrile, butyl or PTFE that acts both as a seal and a valve plug.
These valves can be easily installed into the process line because they are available with various connections including hygienic couplings, flanges and clamp connections according to ISO 2852 and welding ends.
Aseptic control valves
for high purity processes
- by SAMSON AKTIENGESELLSCHAFT
- June 18, 2010
- 770 views