GEMÜ’s single-use diaphragm valve allows a paradigm shift to single-use technology: from manual systems to systems which can be automated for fault-free operation and with complying documentation.
The trend towards simplified plant designs upstream and downstream and the effective prevention of cross-contamination risks is constantly giving an ever more important profile to single-use disposable technology - especially in pharmaceutical process engineering. In the manufacture of small batch sizes up to approx. 2,000 l, which are required in research, and pilot production systems; single-use technology is increasingly being used.
The secondary processes for cleaning and sterilisation (CIP/SIP) that are well-known and required for classic stainless steel material plant designs are in practice no longer necessary at all with single-use plants and processes. The necessary sterility is guaranteed through the sterilization by gamma rays of all the process components which were used. This reduces not only the investment costs of such a plant, but also eliminates the extremely time-consuming cleaning validation for operating media that are no longer required.
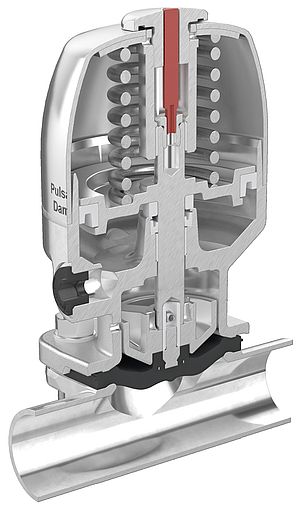
The single-use diaphragm valve with pneumatic actuator has been developed for the increasingly required demand for the automation capability of single-use processes. Valve body and actuator are connected to each other by a patented technology: after the application process, the valve body is disconnected from the actuator, and multiple use is prevented by a specially designed predetermined break point in the valve.
The valve body is manufactured out of special polypropylene in a cleanroom and is sterilizable up to 50 kGy gamma. It isolates the working medium hermetically from the environment and from the actuator through an ultrasonically welded diaphragm. The actuator remains in the system for multiple use. The medium remains closed off from the environment by the sealed diaphragm not only during operation, but also after removing the actuator.
Depending on the process requirements the diaphragm valve body, which is manufactured and packaged in “Low bioburden-quality”, can be delivered directly to the system as a unit with gamma-sterilized tubing and bags.
The single-use diaphragm valve series will at first be offered in two valve body versions in nominal sizes of 3/8"to 1" ASME, with tube tips or Tri Clamp connections. They will be offered as a 2/2-way body for isolation and control of flows, and as a T body, which enables the fully automatable sampling of liquids from an existing system.
ACHEMA 2012 - Single-use diaphragm valves
GEMÜ’s single-use diaphragm valve allows a paradigm shift to single-use technology: from manual systems to systems which can be automated for fault-free operation and with complying documentation.The trend towards simplified plant designs upstream and downstream and the effective prevention of cross-contamination risks is constantly giving an ever more important profile to single-use disposable...
- by Gemü Gebr. Müller Apparatebau
- June 19, 2012
- 829 views