The Pharmaceutical Biotechnology Division of the Fraunhofer Institute of Toxicology and Experimental Medicine (ITEM) has been developing biopharmaceutical substances for 20 years.
The institute received regulatory approval for the production of biopharmaceutical active ingredients in compliance with § 13 of the German Drug Act in 1997. This approval was extended to include the manual aseptic filling of high-volume clinical trial products in April 2010.
By expanding the Pharmaceutical Biotechnology Division on the Helmholtz Center campus for infection research in Braunschweig, Germany, Fraunhofer established the spatial requirements for the sterile filling of small-batch clinical trial drugs.
In accordance with the GMP Guidelines Annex 1 for the aseptic manufacturing of small-batch pharmaceuticals, especially phase I and II clinical trial drugs can now be filled in the laminar flow (LF) area.
The idea: a combination filling machine
The new facility of the Fraunhofer ITEM Pharmaceutical Biotechnology Division in Braunschweig was inaugurated in September 2013. A new cleanroom grade B supports filling operations for investigational drugs in vials and ampoules in very small batch sizes of less than 1,000 to 3,000 pieces.
"With our professional competence that we have accumulated over the past years and our new technology platform, we have now closed the gap between preclinical research and clinical development. We are confident that our current offer will speed up the development of future biopharmaceuticals. In addition, it will be of great interest to the industry, especially for new biopharmaceutical candidates from public research facilities," says Dr. Holger Ziehr, head of Pharmaceutical Biotechnology.
During the inauguration, the Fraunhofer ITEM also presented the new filling and closing machine ARF 1010, which is the result of the first collaboration with Bosch Packaging Technology.
"The Fraunhofer ITEM wants to offer customers quick access to sterile biopharmaceutical trial drugs. Therefore, we needed a solution that operates efficiently, even with the smallest batches and different container types. Our idea was a combination machine that would efficiently fill and close both vials and ampoules, while saving space and conforming to the strict requirements of GMP Annex 1, the German Drug Act, and the German Genetic Engineering Safety Regulations (GenTSV)," Dr. Holger Ziehr concludes.
"We work with active ingredients and had no previous experience with this type of filling operations. We quickly came to realize that there were not many suitable solutions available in the market," says Dr. Luma Baydoun, head of quality control at Fraunhofer ITEM.
At Interpack 2008, the project managers discussed their requirements with Bosch Packaging Technology and laid the foundation for a successful partnership.
The challenge: limited space
The first planning meetings of the new project partners took place in parallel with the reconstruction work at the Braunschweig site.
"A special challenge consisted in the limited space, as the new filling and closing machine needed to be installed in an existing building with a connection to the production machine of the active ingredients," Dr. Ziehr points out.
The overlap in time of the reconstruction work and the discussions with Bosch was advantageous for the project: The new ARF 1010 is now exactly aligned to the new room design, making sure that the space can be optimally used. Through the close collaboration with the Fraunhofer ITEM, the team members derived several design principles such as flexibility, convertibility and space savings.
"It is only with the wooden mock-up model that you can see if concepts developed on the drawing board, in brainstorming sessions or together with users are practicable," explains Tobias Goettler, product manager at Bosch Packaging Technology.
Especially the requirements regarding packaging, pumps, cleanroom classification and protection were worked out in detail; the results were directly transferred to the further developmental work.
"The cooperation between hardware and software developers, operators and model builders was particularly important during the first development phase. Together with the user we verified whether the layout satisfied the daily requirements," explains Tobias Goettler.
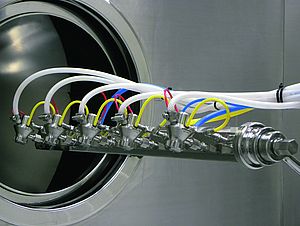
Traditional sterile filling machines are not sufficient when it comes to manufacturing substances such as germs, bacteriophages and biological agents. Fraunhofer's ARF 1010 is equipped with a RABS (Restricted Access Barrier System) and a laminar flow protection cover.
The grade B cleanroom is supplied with 100 per cent fresh air. To decontaminate the biologically active agents, the cleanroom is gassed with hydrogen peroxide (H2O2), which in turn requires a robust machine.
The focus: filling and closing
Due to the spatial restrictions, the Fraunhofer ITEM decided to outsource some of the upstream processes. The traditional aseptic filling operation is divided into three zones: loading and washing, sterilizing and depyrogenazing, filling and closing.
In Braunschweig the focus is on zone three; new partners from the packaging industry were required for the other two zones.
One of them is the northern Italian manufacturer Nuova Ompi, who specializes in pharmaceutical primary packaging made of glass and has cooperated with Bosch in previous projects.
The Fraunhofer ITEM obtains ready-to-fill, sterile and pyrogen-free vials from Nuova Ompi's EZ-fill™ product line. The EZ-fill™ concept steamlines production processes by eliminating the source of potential stress caused to the glass containers, and increases flexibility in filling operations.
The containers with a capacity of ten milliliters are securely packed in a plastic tray when they arrive at the laboratory in Braunschweig.
The tray is sealed with a lid made of tear-proof Tyvek, and is additionally protected through a double Steribag. The pre-sterilized vials are transported from the grade B cleanroom into the RABS via an insert flap, where the operator manually removes the Tyvek packaging using the RABS gloves.
With its single-position hose pump filling system, the filling and closing machine processes 36 vials per minute.
The machine is continuously loaded through an inclined loading station. The containers are singularized and passed on to an intermittent starwheel, where they are transported through the working stations in a particularly gentle and flow-friendly manner.
After the vials have been filled by a peristaltic pump, they are closed and capped in the integrated stoppering station. The empty trays are conveyed to a special container and the unusable vials are transported to the reject station.